Alcedo atthis is the systematic name of a bird otherwise known as common kingfisher or river kingfisher. The names Eurasian kingfisher or European kingfisher hint to the geographic range where Alcedo atthis is living, diving and fishing: in most of Europe and non-Siberian parts of Asia; but also in northern Africa and parts of Australia.
Its German name is Eisvogel, its French name is Martin-pêcheur, and its Spanish name is martín pescador . The adjectives gemein, commun, and común may respectively be added for common.
The kingfisher belongs to the Alcedinidae family (river kingfishers) in the suborder Alcedines of the order Coraciiformes (Ordnung Rackenvögel in German). A northern American relative is, for example, the belted kingfisher (Ceryle alcyon), whose habitats include rivers, lakes, coasts and the Sierra of California.
Keywords: ornithology, Coraciiformes
References
[1] Hannah Holmes: Blaze of Blue • The Eurasian kingfisher is flashy and feisty. National Geographic November 2009, Volume 216, Number 5, pp. 76-85.
[2] Grzimeks Tierleben • Neunter Band • Vögel 3 • Kindler Verlag AG, Zürich, 1970.
Latintos stands for "language transformations in texts and open sources." The LATINTOS BLOG highlights different spellings and different meanings of words, phrases and abbreviations as well as their origin. Latintos compares words in different contexts and different languages including scientific and formal languages. Further, name construction is analyzed and applications of systematic names and nomenclature systems are monitored.
Friday, October 30, 2009
Monday, October 26, 2009
Acronym in chemistry: GDGT for glycerol dibiphytanyl glycerol tetraether
Glycerol dibiphytanyl glycerol tetraethers (GDGTs) are large cyclic tetraether molecules biosynthesized by archaebacteria as membrane building blocks, spanning the membrane lipid layer. Examples of GDGTs are caldarchaeol and crenarchaeol. The latter contains four cyclopentane and one cyclohexane ring within the “large-ring backbone” (see reference [1] and Crenarchaeol, derived from Crenarchaea). The use of biphytanyl glycerol diethers and GDGTs distinguish archaea from other bacteria and eukaryotes. Archaea live in extreme environments and the ether linkages, which are more stable than the ester linkages in membrane lipids of non-archaea, have been interpreted as an adaption to thrive or survive under extreme conditions.
The notation GDGT-n, in which n is an integer specifying the total number of cyclopentane rings in a GDGT molecules, has been introduced [1,2]. Caldarchaeol is a GDGT-0 and crenarchaeol a GDGT-4 compound.
Keywords: biochemistry, thermophilic organisms, pelagic crenarchaeota, membrane architecture
References
[1] J. S. Sinninghe Damsté, S. Schouten, E. C. Hopmans, A. C. T. Duin and J. A. J. Geenevasen: Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeots. J. Lipid Res. 2002, 43, pp. 1641-1651.
DOI: 10.1194/jlr.M200148-JLR200.
[2] J. S. Sinninghe Damsté, W. I. C. Rijpstra, E. C. Hopmans, F. G. Prahl, S. G. Wakeham and S. Schouten: Distribution of Membrane Lipids of Planktonic Crenarchaeota in the Arabian Sea. Appl. Env. Microbiol. 2002, 68, pp. 2997-3002.
DOI: 10.1128/AEM.68.6.2997-3002.2002.
The notation GDGT-n, in which n is an integer specifying the total number of cyclopentane rings in a GDGT molecules, has been introduced [1,2]. Caldarchaeol is a GDGT-0 and crenarchaeol a GDGT-4 compound.
Keywords: biochemistry, thermophilic organisms, pelagic crenarchaeota, membrane architecture
References
[1] J. S. Sinninghe Damsté, S. Schouten, E. C. Hopmans, A. C. T. Duin and J. A. J. Geenevasen: Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeots. J. Lipid Res. 2002, 43, pp. 1641-1651.
DOI: 10.1194/jlr.M200148-JLR200.
[2] J. S. Sinninghe Damsté, W. I. C. Rijpstra, E. C. Hopmans, F. G. Prahl, S. G. Wakeham and S. Schouten: Distribution of Membrane Lipids of Planktonic Crenarchaeota in the Arabian Sea. Appl. Env. Microbiol. 2002, 68, pp. 2997-3002.
DOI: 10.1128/AEM.68.6.2997-3002.2002.
Sunday, October 25, 2009
Crenarchaeol, derived from Crenarchaea
Crenarchaeol derives from Crenarchaea, linguistically and biochemically. Crenarchaea, a taxonomic grouping of archaea, are autotrophic organisms that are widespread in both surface and deep waters of the oceans [1].
Crenarchaeol, a glycerol dibiphytantyl glycerol tetraether (GDGT) that plays a critical role in the membrane structure of planktonic crenarchaea, was named in 2001 by Jaap Sinninghe Damsté of the Netherlands Institute of Sea Research on the North Sea Island of Texel [4-6], when he and colleagues isolated the compound and identified its structure using two-dimensional nuclear magnetic resonance spectroscopy [1]:
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; see, for example, pages 215-217 and glossary.
[2] Jack R. Holt: Introduction to the Kingdom Crenarchaea.
[3] Sue Barnsand Siegfried Burggraf: Crenarchaeota.
[4] J. S. Sinninghe Damsté, S. Schouten, E. C. Hopmans, A. C. T. Duin and J. A. J. Geenevasen: Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeots. J. Lipid Res. 2002, 43, pp. 1641-1651.
DOI: 10.1194/jlr.M200148-JLR200.
[5] J. S. Sinninghe Damsté, W. I. C. Rijpstra, E. C. Hopmans, F. G. Prahl, S. G. Wakeham and S. Schouten: Distribution of Membrane Lipids of Planktonic Crenarchaeota in the Arabian Sea. Appl. Env. Microbiol. 2002, 68, pp. 2997-3002.
DOI: 10.1128/AEM.68.6.2997-3002.2002.
[6] S. Schouten, E. C. Hopmans, E. Schefuß and J. S. Sinninghe Damsté: Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures? Earth Planet Sci. Lett. 2002, 204, pp. 265-274. PDF-version.
Crenarchaeol, a glycerol dibiphytantyl glycerol tetraether (GDGT) that plays a critical role in the membrane structure of planktonic crenarchaea, was named in 2001 by Jaap Sinninghe Damsté of the Netherlands Institute of Sea Research on the North Sea Island of Texel [4-6], when he and colleagues isolated the compound and identified its structure using two-dimensional nuclear magnetic resonance spectroscopy [1]:
It [crenarchaeol] was distinguished from the other ring-containing tetraethers by a biphytanyl chain that had a six-carbon ring in addition to two of the usual five-carbon rings. Damsté christened the compound “crenarchaeol” and, noting that the six-carbon ring created a sort of bulge in the middle of the tetraether, teamed up with theoretical chemists from the University of Amsterdam to create a molecular model of an archaeal membrane structure with the new compound as core lipid. The result offered one possible solution to the apparent paradox of cold water crenarchaea: whereas the five-carbon rings made the biphytanyl chains fit together more snugly in a dense structure, addition of crenarchaeol with its bulging six-carbon ring kept them from packing together too closely and would, conceivably, allow the membrane to maintain its fluidity at low temperatures.References and interesting links
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; see, for example, pages 215-217 and glossary.
[2] Jack R. Holt: Introduction to the Kingdom Crenarchaea.
[3] Sue Barnsand Siegfried Burggraf: Crenarchaeota.
[4] J. S. Sinninghe Damsté, S. Schouten, E. C. Hopmans, A. C. T. Duin and J. A. J. Geenevasen: Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeots. J. Lipid Res. 2002, 43, pp. 1641-1651.
DOI: 10.1194/jlr.M200148-JLR200.
[5] J. S. Sinninghe Damsté, W. I. C. Rijpstra, E. C. Hopmans, F. G. Prahl, S. G. Wakeham and S. Schouten: Distribution of Membrane Lipids of Planktonic Crenarchaeota in the Arabian Sea. Appl. Env. Microbiol. 2002, 68, pp. 2997-3002.
DOI: 10.1128/AEM.68.6.2997-3002.2002.
[6] S. Schouten, E. C. Hopmans, E. Schefuß and J. S. Sinninghe Damsté: Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures? Earth Planet Sci. Lett. 2002, 204, pp. 265-274. PDF-version.
Thursday, October 22, 2009
Acronym in microbiology: ANME for anaerobic methanotrophic archaea
The Linnaean classification system knows only two domains, plants and animals, at the highest level of its hierarchy. With the “birth” of the phylogenetic tree, archaea “arrived” as a third domain.” For details, see, for example, Archaea and the Tree of Life by Preston So.
An interesting set of microorganisms is the group (or supergroup) of anaerobic methanotrophic archaea (ANME), which are described in the glossary of the book Echoes of Life [1] as follows:
Keywords: microbes, taxonomy, phylogenetics
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; see, for example, pages 201-204 and glossary.
An interesting set of microorganisms is the group (or supergroup) of anaerobic methanotrophic archaea (ANME), which are described in the glossary of the book Echoes of Life [1] as follows:
These microorganisms participate in the anaerobic oxidation of methane and incorporate carbon from the methane. At least three phylogenetically distinct groups of ANME have been identified; these appear to be related to several genera of methanogens. ANME are often found living in close association with sulfate-reducing bacteria, sometimes in consortia where methane is oxidized and sulfate reduced.The first discovered “ANME species” was ANME 1—a name that doesn't show much resemblance to the typical binomial nomenclature used within the Linnaean system of taxonomy. With phylogenetics, the concept of a microbe species is loosing sharpness. ANME 1 was discovered and named by microbiologist Ed DeLong and his colleagues at the Monterey Bay Aquarium Research Institute in California, where they studied ribosomal RNA sequences of methanogens found in marine environments and sediments.
Keywords: microbes, taxonomy, phylogenetics
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; see, for example, pages 201-204 and glossary.
Sunday, October 18, 2009
Acronym in analytical chemistry: GC-irm-MS for gas chromatography—isotope ratio monitoring—mass spectrometry
The term gas chromatography—isotope ratio monitoring—mass spectrometry (GC-irm-MS) was coined in the 1980s in Indiana by John Hayes, Kate Freeman and others for a novel technique they applied in the determination of isotopic compositions of compounds in mixtures of environmental samples [1]. The glossary in Echoes of Life gives the following definition of the GC-irm-MS technique [1]:
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; see, for example, page 157 and glossary.
Further references to GC-irm-MS applications and results
[2] The graphical representation on page 158 in [1] gives a nice overview of differences of 13C isotopic composition found in various groups of organisms, the environment and geologic deposits.
[3] Group of 9 authors: Terrestrial vegetation change inferred from n-alkane σ13C analysis in the marine environment. Geochimica et Cosmochimica Acta 1995, 59, pp. 2853-2857.
DOI: 10.1016/0016-7047(95)00160-2.
[4] Bart E. van Dongen, Stefan Schouten and Jaap S. Sinninghe Damsté: Gas chromatography/combustion/isotope-ratio-monitoring mass spectrometeric analysis of methylboronic derivatives of monosaccharides: a new method for determining 13C abundances of carbohydrates. Rapid Communications in Mass Spectrometry 2001, 15 (7), pp. 496-500.
DOI: 10.1002/rcm.259.
A gas chromatograph combined with a combustion interface that burns the separated compounds to CO2, and with a special mass spectrometer that can then measure the relative abundance of isotopes. Used to determine the isotopic composition of individual molecular species.The acronym is sometimes also written in its all-uppercase form GC-IRM-MS. Synonymously, the acronyms irm-GC/MS and GC-IRMS are in use.
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; see, for example, page 157 and glossary.
Further references to GC-irm-MS applications and results
[2] The graphical representation on page 158 in [1] gives a nice overview of differences of 13C isotopic composition found in various groups of organisms, the environment and geologic deposits.
[3] Group of 9 authors: Terrestrial vegetation change inferred from n-alkane σ13C analysis in the marine environment. Geochimica et Cosmochimica Acta 1995, 59, pp. 2853-2857.
DOI: 10.1016/0016-7047(95)00160-2.
[4] Bart E. van Dongen, Stefan Schouten and Jaap S. Sinninghe Damsté: Gas chromatography/combustion/isotope-ratio-monitoring mass spectrometeric analysis of methylboronic derivatives of monosaccharides: a new method for determining 13C abundances of carbohydrates. Rapid Communications in Mass Spectrometry 2001, 15 (7), pp. 496-500.
DOI: 10.1002/rcm.259.
Saturday, October 17, 2009
Emily, a nickname for Emiliania huxleyi
Emily is a short term and nickname that some microbiologists like to use to refer to the coccolithophore Emiliania huxleyi, a species from a group of single-celled marine planktonic algae.
Geologists and paleoceanographers have developed much interest in Emily and related species, since Emiliania huxleyi algae produce long-chain alkenones, of which—when found in marine sediments —the ratio of molecules with different degree of unsaturation (different number of double bonds) can be used to extrapolate to past sea surface temperatures [1].
Keywords: biochemistry, paleoceanography, coccolithophores, molecular structure of unsaturated alkanones, ketones
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; see, for example, page 111 and 114.
Geologists and paleoceanographers have developed much interest in Emily and related species, since Emiliania huxleyi algae produce long-chain alkenones, of which—when found in marine sediments —the ratio of molecules with different degree of unsaturation (different number of double bonds) can be used to extrapolate to past sea surface temperatures [1].
Keywords: biochemistry, paleoceanography, coccolithophores, molecular structure of unsaturated alkanones, ketones
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; see, for example, page 111 and 114.
The term biomarker and its synonyms
The word biomarker was coined in the late 1970s by Wolfgang Seifert at Chevron, when chemical fingerprinting of sediments, crude oil and other samples with organic matter advanced rapidly due to new techniques in analytical chemistry [1]:
From a kinetics viewpoint, biomarkers are organic reaction products (or the molecules thereof)—often decomposition products of much larger biomolecules or supramolecular structures.
Keywords: organic geochemistry, biochemistry, paleontology, history
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; pp. 83-84 and glossary.
[…] Seifert was the one who coined the term “biomarker” from their original “biological marker,” perhaps because it sounded catchier, especially in an industry [oil industry] that was more interested in rocks than biology. The term caught on, even as the work with petroluem biomarkers moved farther and farther from the original concept of a biological marker—from understanding the history of life or the biological origin of hydrocarbons in petroleum, to chronicling the history of rocks and the fate of the hydrocarbons themselves.In the glossary of the book Echoes of Life [1], the term Biomarker is defined as follows:
An organic compound in natural waters, sediments, soils, fossils, crude oils, or coal that can be unambiguously linked to specific precursor molecules made by living organisms.Biological marker, molecular fossil, fossil molecule, and geochemical fossil are given as synonymous expressions for the word biomarker.
From a kinetics viewpoint, biomarkers are organic reaction products (or the molecules thereof)—often decomposition products of much larger biomolecules or supramolecular structures.
Keywords: organic geochemistry, biochemistry, paleontology, history
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; pp. 83-84 and glossary.
Friday, October 16, 2009
Dinosterol, a sterol from dinoflagellates

Dinosterol is one of many sterols that has been isolated from dinoflagellates, a genus of single-celled algae. Dinosterol was identified and named around 1978 when a Dutch group—the research team of organic chemist Jan de Leeuw at Delft University in the Netherlands— studied sterols in Black Sea sediments and another research team (Rhode Island group) analyzed natural products from marine algae that cause “red tides.”
The molecular structure of dinosterol is a biomarker in the paleontological studies of marine sediments. The book Echoes of Life [1] traces the history of the identification of such biomarker molecules. For the chemical compound dinosterol, it gives the following story:
One of its [the Dutch group's] first big successes came […] from a 5000-year-old layer of surface sediments in the Black Sea. Here the group found massive amounts of a sterol with a “peculiar” structure […]. The Black Sea sediments […] were also loaded with the distinctive fossils of dinoflagellates, a large, diverse genus of single-celled algae, and the Dutch group suspected this was the source of the sterol. In 1978, around the same time they determined its structure, a group of Rhode Island natural products chemists who were studying the toxin-producing algae [Gonyaulax tamarensis] responsible for the deadly “red tides” that periodically occur in coastal waters began isolating sterols from dinoflagellates and confirmed de Leeuw's suspicions: the most prevalent of the sterols, which they christened dinosterol, was none other than the Black Sea sterol, a hitherto unknown structure, with a methyl group attached to the A-ring, and and extra branch attached to its side chain.Synonyms for dinosterol (C30H52O), according to the Merck Index, are 4,23-dimethylergost-22-en-3-ol and—no surprise—Black Sea sterol.
Keywords: organic chemistry, sterols, algae, paleontology, history
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; page 108.
Thursday, October 15, 2009
Hopane, a pentacyclic triterpane named—indirectly—after English botanist John Hope
 Hopane and its derivatives (hopanoids) are chemical compounds that occur in certain tropical plants and also in bacterial cell membranes. Further, they have been found in rock and sediment layers that formed in inland lakes and seas, recently—on a paleontological time scale—or during the Eocene. For example, hopanes were identified in samples from Messel and Green River shales.
Hopane and its derivatives (hopanoids) are chemical compounds that occur in certain tropical plants and also in bacterial cell membranes. Further, they have been found in rock and sediment layers that formed in inland lakes and seas, recently—on a paleontological time scale—or during the Eocene. For example, hopanes were identified in samples from Messel and Green River shales.Hopane got its name from a Burmese tree, the Hopea tree, which was named after the British botanist John Hope [1]:
[…] a 30-carbon five-ring affair that people had taken to calling the “hopane” skeleton—not because it inspired particularly positive expectations, but because the genus of Burmese trees where it was first discovered was named after an eighteenth-century English botanist named John Hope. Probably the only reason anyone knew about hopane to begin with was that the British Museum has used resin from Hopea trees as a varnish. What was disconcerting was that compounds with this hopane ring structure were present in every rock or sediment sample one analyzed, no matter when or where it was formed. And yet, as far as anyone knew, such compounds were made only by a few exotic species of tropical trees, ferns, and mosses.The question that remains is Does this Burmese tree species or genus not have a Burmese (Myanmar) name? And if yes, shouldn't the name hopane change into a more Burmese-flavored name? A nightmare for chemical nomenclature!
Anyway, this wouldn't change the molecular structure of hopane (C30H52), a hydrocarbon molecule, which is shown in the sketch above with its carbon numbering and ring lettering.
Keywords: organic chemistry, botany, tree resin, history
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; page 62.
Monday, October 12, 2009
Misleading chemical names during the early days of geochemical studies of oils and shales
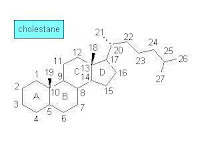 During the 1960s—the early days of GC-MS analysis in chemistry and related disciplines—a large number of natural products, obtained from both biological and geological samples, were systematically analyzed. Among the studied samples were those that included polycyclic organic compounds such as optically active terpenoids. The analytical results were leading to interesting conclusions about fossil materials and their origin while the molecular structures of compounds from living organisms and sedimentary organic matter were compared. Knowledge of the molecular structure of steroids and terpenoids became detailed enough at that time to support such comparative analysis. At first, however, some confusion in terpenoid structure and nomenclature occurred [1]:
During the 1960s—the early days of GC-MS analysis in chemistry and related disciplines—a large number of natural products, obtained from both biological and geological samples, were systematically analyzed. Among the studied samples were those that included polycyclic organic compounds such as optically active terpenoids. The analytical results were leading to interesting conclusions about fossil materials and their origin while the molecular structures of compounds from living organisms and sedimentary organic matter were compared. Knowledge of the molecular structure of steroids and terpenoids became detailed enough at that time to support such comparative analysis. At first, however, some confusion in terpenoid structure and nomenclature occurred [1]:Many of the molecular structures that they [geochemists] puzzled out did indeed resemble the optically active cyclic terpenoids that the natural products chemists were collecting from organisms—except that most of these compounds in the oils and shales had been stripped of their double bonds and oxygen-containing functional groups, reduced, as it were, to their bare carbon skeletons. Excited to find what appeared to be molecular skeletons of known biological steroids and triterpenoids, the geochemists named the hydrocarbons accordingly, and sometimes misleading—precisely because they were missing some of the key information that distinguished the biological compounds. The sterane they called “ergostane” seemed to bear the carbon skeleton of the sterol ergosterol, which is made by fungi and was quite familiar to chemists. But ergosterol differs from brassicasterol only by a double bond in the ring structure, which, of course, was missing from the steranes in the rocks, and the relatively large quantities of ergostane that the geochemists found in in rocks and oils probably had little to do with fungi and everything to do with a certain very productive group of unicellular algae. Likewise, sitosterol, which is plentiful in land plants, and stigmasterol, which is abundant in land plants and algae, are distinguished by an extra double bond in stigmasterol, so one really can't tell whether the 29-carbon sterane that geochemists first called sitostane and later stigmastane derives from land plants or algae or both. Eventually, when the geochemists gave up on trying to distinguish the orientation of the side groups in the fossil molecules, they took to calling them simply 24-methylcholestane and 24-ethylcholestane.The above sketch of the molecular structure of cholestane (C27H48) shows the carbon numbering and ring lettering used in steranes.
Keywords: paleontology, petroleum chemistry, hydrocarbons, steroids, terpenoids, steranes, alkyl-substituted cholestanes, molecular similarity, molecular difference, molecular side chains, functional groups
Reference
[1] Susan M. Gaines, Geoffrey Eglington, and Jürgen Rullkötter: Echoes of Life • What Fossil Molecules Reveal about Earth History. Oxford University Press, New York, 2009; pp. 56-57.
Behind the trademark name: Novec HFE-7100 is a mixture of the two isomers methyl nonafluorobutyl ether and methyl nonafluoroisobutyl ether
Novec™ HFE-7100 is a binary mixture of methyl perfluorobutyl ether constitutional isomers with molecular formula C5H3F9O. The components are:
Related liquids, which have been formulated for applications similar to those of Novec™ HFE-7100, are Novec™ HFE-7200 (ethoxy-nonafluorobutane), Novec™ HFE-71DE (Novec™ HFE-7100 with (E)-1,2-dichloroethene), Novec™ HFE-71DA (Novec™ HFE-7100 with (E)-1,2-dichloroethene and ethanol) and Novec™ HFE-71IPA (Novec™ HFE-7100 with 2-propanol).
- 1-methoxy-nonafluorobutane (also: methyl nonafluorobutyl ether), CF3CF2CF2CF2OCH3, CAS registry number: 163702-07-6, for physicochemical property data see ThermoML references;
- 1-methoxy-nonafluoroisobutane (also: methyl nonafluoroisobutyl ether), (CF3)2CFCF2OCH3, CAS registry number: 163702-08-7.
Related liquids, which have been formulated for applications similar to those of Novec™ HFE-7100, are Novec™ HFE-7200 (ethoxy-nonafluorobutane), Novec™ HFE-71DE (Novec™ HFE-7100 with (E)-1,2-dichloroethene), Novec™ HFE-71DA (Novec™ HFE-7100 with (E)-1,2-dichloroethene and ethanol) and Novec™ HFE-71IPA (Novec™ HFE-7100 with 2-propanol).
Friday, October 9, 2009
Acronym in chemistry: EIL for energetic ionic liquid
By combining the definitions for an ionic liquid and an energetic chemical substance, an energetic ionic liquid (EIL) is an energetic salt with a melting point below 100 °C.
Energetic compounds have a high energy content and are typically applied as fuels or explosives. Naturally, they are hazardous. Methods that allow derivatization of molecular energetic compounds into ionic ones have been suggested to provide safer routes to better manageable energetic materials.
Energetic ionic compounds such as ammonium, hydrazonium, and guanidinium salts are known for quite some time. Current design of EILs considers cations and anions that both have a high nitrogen content, typically two to four nitrogen atoms as heteroatoms in a five-membered aromatic ring with alkyl side chains as well as azido, nitro or cyano groups as substituents. The anions may also by acyclic ions such as nitrate, halides or perchlorate.
Keywords: advanced materials, molecular design, ionic liquid design, physical and chemical properties, hazardous materials, material safety
Selected literature
[1] Marcin Smigla, Andreas Metlen, and Robin D. Rogers: The Second Evolution of Ionic Liquids: From Solvents and Separations to Advanced Materials—Energetic Examples from the Ionic Liquid Cookbook. Acc. Chem. Res. 2007, 40, pp. 1182-1192.
DOI: 10.1021/ar7001304.
[2] Arindrajit Chowdhury, Stefan T. Thynell and Ping Lin: Confined rapid thermolysis/FTIR/ToF studies of tetrazolium-based energetic ionic liquids. Thermochim. Acta 2009, 485, pp. 1-3.
DOI: 10.1016/j.tca.2008.11.018.
[3] Cesar Cadena: Molecular Modeling of the Thermophysical and Transport Properties of Ionic Liquids. Dissertation, University of Notre Dame, Indiana, Sept. 2006. PDF-version.
Open access to thermodynamic data (ThermoML-encoded) for ionic liquids including EILs:
http://www.axeleratio.com/thmlbib/main/chemindexes.htm
Energetic compounds have a high energy content and are typically applied as fuels or explosives. Naturally, they are hazardous. Methods that allow derivatization of molecular energetic compounds into ionic ones have been suggested to provide safer routes to better manageable energetic materials.
Energetic ionic compounds such as ammonium, hydrazonium, and guanidinium salts are known for quite some time. Current design of EILs considers cations and anions that both have a high nitrogen content, typically two to four nitrogen atoms as heteroatoms in a five-membered aromatic ring with alkyl side chains as well as azido, nitro or cyano groups as substituents. The anions may also by acyclic ions such as nitrate, halides or perchlorate.
Keywords: advanced materials, molecular design, ionic liquid design, physical and chemical properties, hazardous materials, material safety
Selected literature
[1] Marcin Smigla, Andreas Metlen, and Robin D. Rogers: The Second Evolution of Ionic Liquids: From Solvents and Separations to Advanced Materials—Energetic Examples from the Ionic Liquid Cookbook. Acc. Chem. Res. 2007, 40, pp. 1182-1192.
DOI: 10.1021/ar7001304.
[2] Arindrajit Chowdhury, Stefan T. Thynell and Ping Lin: Confined rapid thermolysis/FTIR/ToF studies of tetrazolium-based energetic ionic liquids. Thermochim. Acta 2009, 485, pp. 1-3.
DOI: 10.1016/j.tca.2008.11.018.
[3] Cesar Cadena: Molecular Modeling of the Thermophysical and Transport Properties of Ionic Liquids. Dissertation, University of Notre Dame, Indiana, Sept. 2006. PDF-version.
Open access to thermodynamic data (ThermoML-encoded) for ionic liquids including EILs:
http://www.axeleratio.com/thmlbib/main/chemindexes.htm
Tuesday, October 6, 2009
Acronym in physics and chemistry: SHE for super heavy elements
SHE is in use as an acronym for the term super heavy elements (also written in two-word form: superheavy elements).
Uranium with atomic number 92 is a typical heavy element. To belong to the SHE set, an element needs to have a higher atomic number. Isotopes with atomic number 93 and higher are radioactive and instable—some with an extremely short life span.
SHE have super heavy atoms and, consequently, super heavy nuclei. The super heavy nucleus 298114, containing 114 protons and 184 neutrons, is often referred to as the doubly magic spherical nucleus. For nuclei in the 298114 neighborhood, enhanced nuclear stability has been predicted. The element with atomic number number 112, for which the name copernicium has been proposed, falls into this neighborhood. The term super heavy elements or its acronym SHE is most often used in the context of chemical elements that feature isotopes within this neighborhood, in which they establish islands of stability.
Uranium with atomic number 92 is a typical heavy element. To belong to the SHE set, an element needs to have a higher atomic number. Isotopes with atomic number 93 and higher are radioactive and instable—some with an extremely short life span.
SHE have super heavy atoms and, consequently, super heavy nuclei. The super heavy nucleus 298114, containing 114 protons and 184 neutrons, is often referred to as the doubly magic spherical nucleus. For nuclei in the 298114 neighborhood, enhanced nuclear stability has been predicted. The element with atomic number number 112, for which the name copernicium has been proposed, falls into this neighborhood. The term super heavy elements or its acronym SHE is most often used in the context of chemical elements that feature isotopes within this neighborhood, in which they establish islands of stability.
Monday, October 5, 2009
The name ‘copernicium’ has been suggested for the chemical element with atomic number 112
In a recent interview with Nachrichten aus der Chemie [1], physicist Sigurd Hofmann of the Center for Heavy Ion Research (GSI Helmholtz-Zentrum für Schwerionenforschung) in Darmstadt, Germany, whose international group (a total of 21 scientists) discovered and produced (a few atoms of) the element 112 in 1996, explains, why they proposed the name copernicium. He says that many names of theoretical as well as experimental physicists, who did outstanding work in atomic and nuclear physics, have already been taken and are present in the Periodic Table of Elements: for example, Nils Bohr with bohrium (element 107 with symbol Bh), Lise Meitner with meitnerium (element 109 with symbol Mt), or Wilhelm Conrad Röntgen with roentgenium (element 111 with symbol Rg). Hofman continues that this time his group wanted to go further back in history. They came across Nicolaus Copernicus, who, at the end of the middle ages and the beginning of modern times, significantly influenced the way in which we see the world (universe) today.
Symbols Cn and Cp have been proposed for element 112, assuming copernicium is going to become its name. A decision by IUPAC, which would finalize the acceptance of copernicium as the systematic element name, can be expected in 2010. Until then, the temporary name ununbium with the three-letter symbol Uub continues to be the name for this transition metal, for which isotopes with half-lifes (half-lives) in the seconds and milliseconds range have been synthesized. If synthesized in substance amount, ununbium is predicted to be liquid at room temperature, exhibiting similarity with mercury (element 80 with symbol Hg). Mercury is also known as quicksilver and shares—in the element coordination of the Periodic Table— a diagonal relationship with silver. Since the chemical element gold (Au) and ununbium also show such a diagonal relation, quickgold makes an analogical name alternative for element 112—just for the unlikely case that copernicium does not come through.
References
[1] Element 112: Ich trage einen großen Namen. Nachrichten aus der Chemie September 2009, Volume 57, page 851.
[2] ‘Copernicium’ Proposed As Name For Newly Discovered Element 122 (Science Daily) .
[3] Welcome ‘Copernicium,’ our Newest Element (Universe Today).
[4] Periodic Table of Elements including element 112 (Table annotations in German, symbols of chemical elements international).
[5] R. Eichler, W. Brüchle, R. Buda, S. Bürger, R. Dressler, Ch. E. Düllmann, J. Dvorak, K. Eberhardt, B. Eichler, C. M. Folden III, H. W. Gäggeler, K. E. Gregorich, F. Haenssler, D. C. Hoffman, H. Hummerich, E. Jäger, J. V. Kratz, B. Kuczewski, D. Liebe, D. Nayak, Nitsche, D. Piguet, Z. Qin, U. Rieth, M. Schädel, B. Schausten, E. Schimpf, A. Semchenkov, S. Soverna, R. Sudowe, N. Trautmann, P. Thörle, A. Türler, B. Wierczinski, N. Wiehl, P. A. Wilk, G. Wirth, A. B. Yakushev and A. von Zweidorf: Attempts to chemically investigate element 112. Radiochim. Acta 2006, 94, 181-191. DOI: 10.1524/ract.2006.94.4.181.
[6] R. C. Barber, H. W. Gäggeler; P. J. Karol, H. Nakahara, E. Vardaci and E. Vogt: Discovery of the Element with Atomic Number 112 (IUPAC Technical Report). Pure Appl. Chem. 2009, 81 (7), 1331-1343.
DOI: 10.1351/PAC-REP-08-03-05.
Symbols Cn and Cp have been proposed for element 112, assuming copernicium is going to become its name. A decision by IUPAC, which would finalize the acceptance of copernicium as the systematic element name, can be expected in 2010. Until then, the temporary name ununbium with the three-letter symbol Uub continues to be the name for this transition metal, for which isotopes with half-lifes (half-lives) in the seconds and milliseconds range have been synthesized. If synthesized in substance amount, ununbium is predicted to be liquid at room temperature, exhibiting similarity with mercury (element 80 with symbol Hg). Mercury is also known as quicksilver and shares—in the element coordination of the Periodic Table— a diagonal relationship with silver. Since the chemical element gold (Au) and ununbium also show such a diagonal relation, quickgold makes an analogical name alternative for element 112—just for the unlikely case that copernicium does not come through.
References
[1] Element 112: Ich trage einen großen Namen. Nachrichten aus der Chemie September 2009, Volume 57, page 851.
[2] ‘Copernicium’ Proposed As Name For Newly Discovered Element 122 (Science Daily) .
[3] Welcome ‘Copernicium,’ our Newest Element (Universe Today).
[4] Periodic Table of Elements including element 112 (Table annotations in German, symbols of chemical elements international).
[5] R. Eichler, W. Brüchle, R. Buda, S. Bürger, R. Dressler, Ch. E. Düllmann, J. Dvorak, K. Eberhardt, B. Eichler, C. M. Folden III, H. W. Gäggeler, K. E. Gregorich, F. Haenssler, D. C. Hoffman, H. Hummerich, E. Jäger, J. V. Kratz, B. Kuczewski, D. Liebe, D. Nayak, Nitsche, D. Piguet, Z. Qin, U. Rieth, M. Schädel, B. Schausten, E. Schimpf, A. Semchenkov, S. Soverna, R. Sudowe, N. Trautmann, P. Thörle, A. Türler, B. Wierczinski, N. Wiehl, P. A. Wilk, G. Wirth, A. B. Yakushev and A. von Zweidorf: Attempts to chemically investigate element 112. Radiochim. Acta 2006, 94, 181-191. DOI: 10.1524/ract.2006.94.4.181.
[6] R. C. Barber, H. W. Gäggeler; P. J. Karol, H. Nakahara, E. Vardaci and E. Vogt: Discovery of the Element with Atomic Number 112 (IUPAC Technical Report). Pure Appl. Chem. 2009, 81 (7), 1331-1343.
DOI: 10.1351/PAC-REP-08-03-05.